Research News
Beyond the Crystal: Capturing Loop Flexibility in PRRSV Drug Design
A structure-based approach reveals baicalin as a promising repurposed entry inhibitor.
Porcine reproductive and respiratory syndrome virus (PRRSV) continues to devastate the global swine industry, yet the structural basis of how small molecules block its entry into host cells remains unclear. Researchers at University of Tsukuba and Mahidol University developed a refined model of the PRRSV receptor domain CD163-SRCR5 using state-of-the-art computational approaches, offering new avenues for rational drug design.
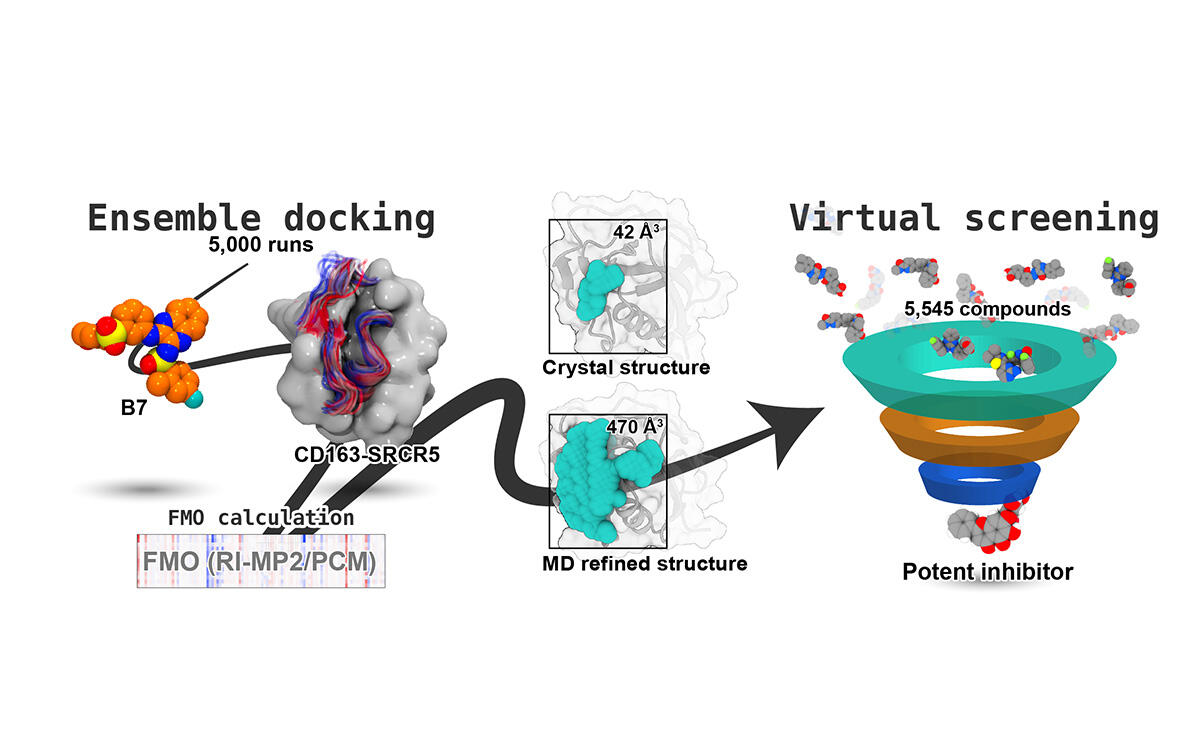
Tsukuba, Japan—While traditional drug discovery often relies on static crystal structures, many biologically important proteins, including the scavenger receptor CD163-SRCR5, contain flexible loop regions poorly captured by crystallography. These loops are critical for recognizing ligands and viral proteins, making them challenging yet attractive drug targets. In this study, researchers used molecular dynamics (MD) simulations, ensemble docking, and fragment molecular orbital calculations to generate a dynamic, physiologically relevant structural model of the CD163-SRCR5 domain.
The MD-refined model, designated p5-343, revealed a novel groove-like pocket not visible in the crystal structure, enabling more accurate prediction of small-molecule binding. The team conducted virtual screening of a repurposing compound library and identified baicalin, a flavonoid with known antiviral properties, as the top candidate. Baicalin showed stable binding and favorable energetics, consistent with previous experimental reports.
This flexible-receptor docking framework is not limited to PRRSV. It can be broadly applied to other therapeutically relevant systems with intrinsically disordered regions or loop-dominated binding interfaces, such as viral proteins, membrane receptors, and host-pathogen complexes. These findings offer a powerful computational solution for structure-based drug discovery beyond conventional targets.
###
This research was supported by JSPS KAKENHI Grant Numbers 21H05269 and 24K20888, the JST-CREST project (Grant Number JPMJCR20B3), and the Faculty of Science, Mahidol University.
Original Paper
- Title of original paper:
- Mechanistic Insights into PRRSV Inhibition through CD163-SRCR5 Blockade by PRRSV/CD163-IN‑1
- Journal:
- The Journal of Physical Chemistry Letters
- DOI:
- 10.1021/acs.jpclett.5c01528
Correspondence
Assistant Professor HENGPHASATPORN Kowit
Professor SHIGETA Yasuteru
Center for Computational Sciences, University of Tsukuba
Related Link
Center for Computational Sciences


